G-MESH®
The G-MESH range of gynecological meshes was awarded a gold medal at the Inventica 2019 fair in Romania.
Product description:
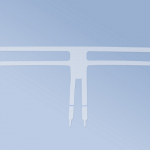
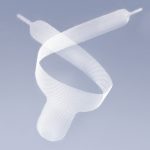
G-MESH® - non-resorbable, lightweight gynecological mesh for use in reconstructive surgery for pelvic organ prolapse (POP), made using the knitting technique of transparent and blue polypropylene monofilament yarn with a high degree of tolerance to the body. Mesh with a macroporous structure.
It is offered in three variants:
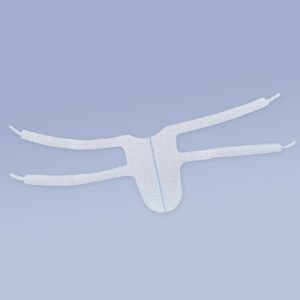
- Posterior 2 - two-arm mesh intended for posterior plasty (rectocele, enterocele)
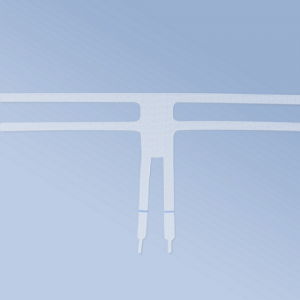
- Anterior 4 - a four-arm mesh intended for anterior plasty (cystocele)
- Posterior 6 - a six-arm mesh intended for posterior plasty (rectocele, enterocele). The Posterior 6 variant is also recommended for patients with uterine prolapse or vaginal diverticulum.
The product is characterized by:
- low surface mass,
- atraumatic shores,
- high stability of the mesh fabric,
- high elasticity and biostability,
- lack of irritation and sensitization,
- the blue orientation line running through the center of the implant facilitates its correct location in tissue structures.
Application:
G-MESH® urgical meshes are used for reconstructive surgery to treat pelvic organ prolapse. Reconstructive surgery should not be the first choice of treatment and should be dedicated to patients with symptomatic pelvic organ prolapse. Treatment is only possible if the patient or legal guardian is aware of the possible complications and agrees to surgical treatment. The amount of mesh used should be limited as much as possible. Surgical therapy should be performed in accordance with the European Association of Urology guidelines.
Sterilization:
G-MESH® gynecological meshes are offered for sale as a sterile material – sterilized with ethylene oxide (EO) in a validated process. The product maintains sterility within the specified shelf life only in the original packaging, when maintaining appropriate storage conditions.
Product description:
G-MESH® - non-resorbable, lightweight gynecological mesh for use in reconstructive surgery for pelvic organ prolapse (POP), made using the knitting technique of transparent and blue polypropylene monofilament yarn with a high degree of tolerance to the body. Mesh with a macroporous structure.
It is offered in three variants:
- Posterior 2 - two-arm mesh intended for posterior plasty (rectocele, enterocele)
- Anterior 4 - a four-arm mesh intended for anterior plasty (cystocele)
- Posterior 6 - a six-arm mesh intended for posterior plasty (rectocele, enterocele). The Posterior 6 variant is also recommended for patients with uterine prolapse or vaginal diverticulum.
The product is characterized by:
- low surface mass,
- atraumatic shores,
- high stability of the mesh fabric,
- high elasticity and biostability,
- lack of irritation and sensitization,
- the blue orientation line running through the center of the implant facilitates its correct location in tissue structures.
Application:
G-MESH® urgical meshes are used for reconstructive surgery to treat pelvic organ prolapse. Reconstructive surgery should not be the first choice of treatment and should be dedicated to patients with symptomatic pelvic organ prolapse. Treatment is only possible if the patient or legal guardian is aware of the possible complications and agrees to surgical treatment. The amount of mesh used should be limited as much as possible. Surgical therapy should be performed in accordance with the European Association of Urology guidelines.
Sterilization:
G-MESH® gynecological meshes are offered for sale as a sterile material – sterilized with ethylene oxide (EO) in a validated process. The product maintains sterility within the specified shelf life only in the original packaging, when maintaining appropriate storage conditions.