Interview with Tricomed’s President of the Board, Witold Sujka, in “Przegląd Włókienniczy”
The latest issue of “Przegląd Włókienniczy” includes the interview with Tricomed’s President of the Board, Witold Sujka. The article covers issues such as the company, its Employees and our latest successes.
Article available under the links (Polish version only)
TRICOMED in “Przegląd Włókienniczy” as a result of Technical Textile Products Fair
“Przegląd Włókienniczy – włókno, odzież, skóra” (issue: 11/2016) decided to include TRICOMED in their report from this year edition of Technical Textile Products Fair. The article described the success of our new ultralight meshed. Also our Codubix Orbital Wall 3D implant got covered by the article.
The article to be found under the links:
Tricomed awarded during III Technical Textile Products Fair!
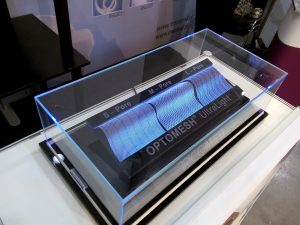
Between 12-13 of October 2016 the 3rd Technical Textile Products Fair was taking place. Tricomed was not only present during the Fair, but we also took part in the InnovaTex Conference. Our two new products were presented during the event: Optomesh ULTRALIGHT hernia meshes and Codubix Orbital Wall 3D anatomical orbital wall implants. Both products earned high esteem of the Visitors and our mesh implants were awarded with the 1st prize for most innvative product.
ITC&DC – INTERNATIONAL TEXTILE, CLOTHING & DESIGN CONFERENCE
Tricomed SA takes part in ITC&DC – INTERNATIONAL TEXTILE, CLOTHING & DESIGN CONFERENCE, this year titled “Magic World of Textiles”. The Conference takes place 2-5.10.2016 in Dubrovnik, Croatia.
More info available at: http://itcdc.ttf.unizg.hr/
Codubix® Orbital Wall 3D prosthesis
Anatomical, three-dimensional implant of mid-facial bone defects is intended for reconstruction of orbital wall fractures (zygomaticomaxillary-orbital fractures, isolated fractures of orbital floor)caused by mid-facial skull bone fractures resulting from trauma or other causes.
New surgical meshes on offer!
Surgical meshes
Optomesh ULTRALIGHT
The Optomesh ULTRALIGHT meshes are the newest generation of mesh implants used in abdominal hernia treatment.
Optomesh ULTRALIGHT L-Pore: mesh with pore size of 6 mm2 and ultralight surface mass 24 g/m2, the following parameters make the mesh especially soft, elastic and easy to handle in surgical field. Big pores allow an appropriate overgrowth of scar tissue without the bridging effect. The implant stays soft even long time after the implantation. The products are characterised with optimal mechanical properties both in longitudinal and transverse direction (above 16N/cm).
Optomesh ULTRALIGHT M-Pore: the mesh is characterised by low surface mass (36 g/m2) and, at the same time, very high mechanical resistance parameters both in longitudinal and transverse direction (above 32 N/cm). The pore size above 4mm ensures optimal overgrowth with connective tissue, which eliminates the problem of chronic inflammation.
Optomesh ULTRALIGHT S-Pore: a universal mesh knitted of yarn with 0,08 mm diameter which ensures elasticity and softness of the mesh. It has atraumatic edges, easy intraoperative handling and it retains its high mechanical properties both in longitudinal and transverse direction (20-30N/cm). The mesh has ultralight surface mass (34 g/m2). Moreover, pore size above 1mm ensures optimal overgrowth with connective tissue, which eliminates the problem of chronic inflammation.
All meshes can be easily cut during the surgery without the need of using specialised tools. Owing to carefully chosen weave, the products do not crumble or fray while cutting. The blue orienting lines in L-Pore and S-Pore variants allow for easy identification of the mesh and the direction of its placement in the surgical field, which facilitates mesh placement in tissue structures.
Intended use:
Optomesh ULTRALIGHT surgical meshes are intended for reconstruction procedures in order to reconstruct and/or strengthening soft tissue defects in the following cases of hernia:
- primary and recurrent abdominal hernia
- large gate post-operative hernias,
- inguinal hernias,
- periumbilical hernias,
- hernias in post-operative scar.
Depending on the hernia type and the preferred surgical method, an appropriate mesh type can be chosen:
- Optomesh ULTRALIGHT L-Pore type is recommened for hernias with small connective tissue defects (hernia gate diameter up to 5 cm);
- Optomesh ULTRALIGHT M-Pore type can be used in small, medium and larg hernias (hernia gate diameter up to 10 cm and more);
- Optomesh ULTRALIGHT S-Pore type is recommended for small and medium-sized hernias (hernia gate diameter up to 10 cm).
Optomesh ULTRALIGHT meshes can be used for tension-free, open surgical techniques (the implant in onlay position) and in laparoscopic techniques (the implants in sublay position).
Characteristic features of ultralight meshes
| Optomesh ULTRALIGHT | yarn | yarn diameter[mm] | pore size [mm2] | surface mass [g/m2] | breaking strength [N/cm] |
| L-Pore | polipropylene | 0,1 | 6 | 24 | >16 |
| M-Pore | polipropylene | 0,1 | 3 | 36 | >32 |
| S-Pore | polipropylene | 0,08 | 2 | 34 | 20 – 30 |
New products from Medisorb P Plus family
Soon our offer will be extended with new products from Medisorb P Plus absorptive dressings family!
New products are characterised by easy application method. The Medisorb P Plus Adhesive has an adhesive layer, the Medisorb P Plus Border dressing is equipped with adhesive occlusive layer. Owing to this, there is no need of using additional fixation dressings. Medisorb P Plus Adhesive and Medisorb P Plus Border are intended to be used in the treatment of exuding wounds, burns and ulcerations.
Both product obtained CE safety mark are being introduced into the market.
Tilene Guard and Tilene Guard Set titanised mesh implants available in Poland
Tilene Guard and Tilene Guard Set titanised mesh implants for parastomal hernia available in Tricomed offer for Poland exclusively.
More information available on the Manufacturer’s site: http://www.pfmmedical.com/en/productcatalogue/mesh_implants_hernia_surgery/tileneR_guard/index.html
Tricomed at Technical Textile Products Fair

Between 12-13 of October 2016 Tricomed will be present at Technical Textile Products Fair and will take active part in InnovaTex conference. The event will be held in Lodx Expo Hall
IV National Conference “Polish Surgery”
Tricomed SA takes part in IV National Conference ‘Polish Surgery – evolution of mode of action in search of further development’.
More information available at: http://polskachirurgia2016.agora-konferencje.pl/pl/program
 Pobierz Pobierz | IV Ogólnopolska Konferencja Polska Chirurgia |